A Proven Non-drug Treatment
Many Benefits of TMS Therapy
- FDA cleared
- Non-drug
- Non-invasive
- No side effects of drugs
- Not ECT
- Long lasting relief
- Covered by insurance
- Proven to work
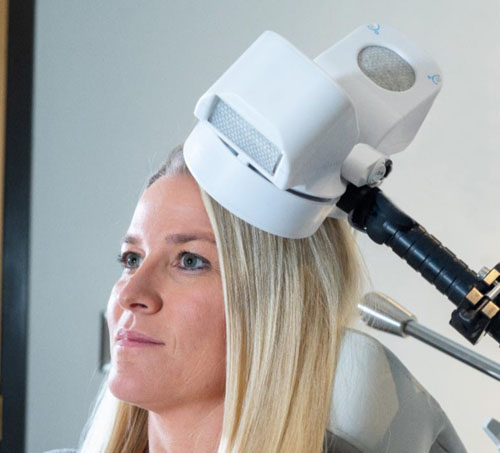
Advantage TMS uses the Apollo TMS Therapy System, which delivers the most effective TMS stimulation in the industry.
Magnetic energy similar to what is used in an MRI is used to stimulate the areas of the brain that are sluggish and causing depressed mood.
TMS has been FDA cleared since 2008 and Apollo gives you the best TMS treatment available.
Clinical trials have demonstrated the safety and effectiveness of TMS Therapy in treating patients who have not benefited from prior antidepressant medication. TMS Therapy was studied in adult patients suffering from Major Depressive Disorder, all of whom had not received satisfactory improvement with previous treatments.
TMS stands for transcranial magnetic stimulation. It is used to treat depression by stimulating the brain non-invasively using electromagnetic fields, similar to those produced by an MRI machine. During TMS Therapy, a magnetic field is administered in very short pulses to the part of the brain that research has demonstrated to be associated with depression. The typical initial course of treatment is about 19-37 minutes daily over 6-7 weeks.
TMS Therapy uses short pulses of magnetic fields to stimulate the area of the brain that functions abnormally in patients with depression. The magnetic field produces an electric current in the brain that stimulates the brain cells (neurons). This results in changes that are beneficial in the treatment of depression.
It usually takes time for healthcare insurers to establish coverage policies for newly approved treatments such as TMS. However, many commercial plans have recognized the effectiveness of treating depression with TMS Therapy and now cover TMS as part of their plans.
TMS is non-systemic (does not circulate in the blood throughout the body), so it does not have side effects such as weight gain, sexual dysfunction, nausea, dry mouth, sedation, etc. The most common side effects reported during clinical trials were headache and scalp discomfort – generally mild to moderate – occurring less frequently after the first week of treatment.
No. TMS therapy involves a unique method of using pulsed magnetic fields for therapeutic benefit. The intensity of the magnetic field is similar to that of the magnetic fields used in magnetic resonance imaging, or MRI. These techniques differ radically from the popular use of low intensity, static magnetic fields. Those products deliver weak and undirected static fields that are not capable of activating brain cells.
© 2024 Advantage Mental Health. All Rights Reserved.
Our office is closed Wednesday, October 9 and Thursday, October 10, 2024, due to the storm. If you have any questions or urgent requests, please email us at info@advantagementalhealth.com.
Please fill out the form if you have a patient interested in learning more about TMS treatment. We appreciate the referral and partnership.
Next Steps:
• Our TMS Coordinator will contact the patient to schedule a complimentary information session.
• If the patient decides to seek TMS treatment with Advantage TMS, we will communicate with you when necessary and the patient will continue to work with you while receiving treatment.
Questions?
• Please don’t hesitate to contact us at 727-600-8093 or info@advantagementalhealth.com
